The Hartree equations were derived by considering the electronic
wavefunction as a product of many single particle wavefunctions,
![]() , each subject to its own external potential. Thus
, each subject to its own external potential. Thus
The Schrödinger equation for each single particle state, ![]() ,is given by
,is given by
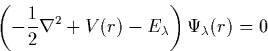 |
(3) |
Hartree suggested that in a many electron system it was possible to replace V(r) with Veff(r), which included the mean electrostatic potential from all of the electrons [13]. This leads to the Hartree self-consistent equations
The total many body wavefunction is then formed as the product of
these one-electron spin-orbitals. V(r) is the potential due to the
ions. This simple approximation leads to surprisingly accurate
results, but suffers from the lack of electronic exchange, i.e.
the many body wavefunction, ![]() is not antisymmetric with
respect to a switching of two of the electrons. This is in breach of
the Pauli principle, which in general imposes a change of sign of the
total wavefunction when two electrons are switched, and in the
independent electron approximation described above, does not allow
more than one electron to occupy a given state.
is not antisymmetric with
respect to a switching of two of the electrons. This is in breach of
the Pauli principle, which in general imposes a change of sign of the
total wavefunction when two electrons are switched, and in the
independent electron approximation described above, does not allow
more than one electron to occupy a given state.