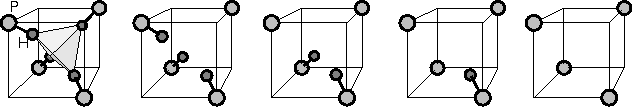
 |
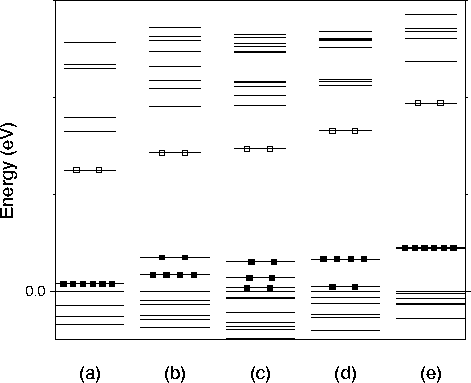 |
As each hydrogen atom is removed from the vacancy, an electron is
removed from the highest occupied state. The resulting defects can act
as acceptors that fill the t2 -level when ionised. This level is
already filled in the case of ![]() so this defect is
electrically neutral. However
so this defect is
electrically neutral. However ![]() ,
, ![]() and
and ![]() will behave as single, double and triple acceptors
respectively.
will behave as single, double and triple acceptors
respectively.
The t2-level is split due to the lower symmetry of the partially
hydrogenated vacancies. ![]() has Td symmetry and
therefore the level does not split.
has Td symmetry and
therefore the level does not split. ![]() has C3v
symmetry, so the t2 splits into an e- and a1- level.
has C3v
symmetry, so the t2 splits into an e- and a1- level. ![]() has C2v symmetry which leads to three separated
singlets.
has C2v symmetry which leads to three separated
singlets. ![]() and
and ![]() are directly
comparable with
are directly
comparable with ![]() and
and ![]() , with the
dangling and hydrogen terminated bonds reversed, in addition to the
a - e level ordering. This t2-like state gradually moves
upwards away from the valence band as hydrogen atoms are removed. In
, with the
dangling and hydrogen terminated bonds reversed, in addition to the
a - e level ordering. This t2-like state gradually moves
upwards away from the valence band as hydrogen atoms are removed. In
![]() the t2-level is quite deep in the gap.
the t2-level is quite deep in the gap.
The indium vacancy, ![]() , fits the trend of increasing
acceptor character with decreasing number of hydrogen atoms present.
Previous calculations [68] also show
, fits the trend of increasing
acceptor character with decreasing number of hydrogen atoms present.
Previous calculations [68] also show ![]() to be a
triple acceptor.
to be a
triple acceptor.
The local vibrational modes for ![]() with pure and mixed
isotope composition are shown in Table 4.3. The highest IR
visible mode for
with pure and mixed
isotope composition are shown in Table 4.3. The highest IR
visible mode for ![]() at 2356.4 cm-1 is in excellent
agreement with experiment (2315.6 cm-1, a 1.8% error), as is the
1690.8 cm-1 mode for
at 2356.4 cm-1 is in excellent
agreement with experiment (2315.6 cm-1, a 1.8% error), as is the
1690.8 cm-1 mode for ![]() (experimental value of
1683.4 cm-1, an error of 0.4%). This small drop in error with
deuteration suggests only limited anharmonic character in the bonding
[37].
(experimental value of
1683.4 cm-1, an error of 0.4%). This small drop in error with
deuteration suggests only limited anharmonic character in the bonding
[37].
| H4 | H3D | H2D2 | HD3 | D4 |
| * 2387.85 | 2380.18 | 2372.34 | 2364.31 | * 1713.01 |
| T 2356.40 | D 2356.32 | 2356.24 | 1707.26 | T 1690.82 |
| D 618.57 | 1696.43 | 1701.74 | D 1690.95 | D 445.34 |
| T 565.87 | D 603.12 | 1691.08 | D 549.65 | T 411.94 |
| T 408.69 | 565.71 | 595.02 | D 428.92 | |
| D 508.72 | 556.04 | 412.03 | ||
| 408.69 | 541.80 | |||
| 481.28 | ||||
| 425.83 |
The LVMs for the range of hydrogenated vacancies, ![]() are shown in Table 4.4. The results show that as the
number of H atoms in the vacancy increases, the P-H bonds are shorten
and the vibrational modes increase. This is due to the compressive
effect of the other hydrogen atoms on each P-H group, coupled with the
removal of dangling bonds from `unsaturated' P atoms which would have
acted to attract the hydrogen away from its phosphorus neighbour.
This is consistent with previous results obtained for Si
[60,69].
are shown in Table 4.4. The results show that as the
number of H atoms in the vacancy increases, the P-H bonds are shorten
and the vibrational modes increase. This is due to the compressive
effect of the other hydrogen atoms on each P-H group, coupled with the
removal of dangling bonds from `unsaturated' P atoms which would have
acted to attract the hydrogen away from its phosphorus neighbour.
This is consistent with previous results obtained for Si
[60,69].
| Defect | 5cLocal Vibrational Modes | Symmetry | Bond | ||||
| Exp [61] | [59] ([70]) | 3cCalculation | Length | ||||
| 2315.2 | 2315.6 | 2387.8* | 2356.4T | 618.6D | Td | 1.419 | |
| 1683.4 | 1713.0* | 1690.8T | 445.3D | ||||
| 2324.1 | 2286.3D | 695.3D | C3v | 1.429 | |||
| 1667.6 | 1640.8D | 498.2D | |||||
| 2256.2 | 2216.8 | 730.0 | C2v | 1.439 | |||
| 617.7 | 611.8 | 602.0 | |||||
| 1619.4 | 1591.6 | 523.0 | |||||
| 446.4 | 444.4 | 438.8 | |||||
| 2201.7 | (2202.4) | 2150.7 | 644.9 | 644.7 | C3v | 1.450 | |
| 1603.8 | 1544.7 | 467.5 | 467.4 |
The shift in P-H length from ![]() to
to ![]() is only
2.1% (from 1.419 Å to 1.450 Å), but it leads to a 9.9% shift in
vibrational mode (2387.8 cm-1 to 2150.7 cm-1). The
calculated H- stretch modes of the partially hydrogenated vacancies
could account for a group of experimentally observed vibrational modes
lying between 2200 and 2290 cm-1 [59,70].
is only
2.1% (from 1.419 Å to 1.450 Å), but it leads to a 9.9% shift in
vibrational mode (2387.8 cm-1 to 2150.7 cm-1). The
calculated H- stretch modes of the partially hydrogenated vacancies
could account for a group of experimentally observed vibrational modes
lying between 2200 and 2290 cm-1 [59,70].
High temperature annealing of InP:Fe reduces the concentration of ![]() . This is due to the partial dissociation of this centre
and, up until now, only
. This is due to the partial dissociation of this centre
and, up until now, only ![]() has been identified with
certainty. It is shown here that
has been identified with
certainty. It is shown here that ![]() and
and ![]() are
acceptors. In annealed high-resistivity material they should cause a
drop in the
are
acceptors. In annealed high-resistivity material they should cause a
drop in the ![]() concentration. This will be in addition to
the decrease in concentration of
concentration. This will be in addition to
the decrease in concentration of ![]() due to loss of the
due to loss of the ![]() donor.
donor.
In contrast, Bardeleben et al [71] found that
thermal annealing of Fe doped InP in the range 660-820 ![]() C led
to an increase in
C led
to an increase in ![]() concentration. They suggested this was
due to the formation of some unidentified deep thermal donors. This
could be associated with other hydrogen complexes. However, from this
work it seems unlikely that hydrogenated vacancies are responsible.
concentration. They suggested this was
due to the formation of some unidentified deep thermal donors. This
could be associated with other hydrogen complexes. However, from this
work it seems unlikely that hydrogenated vacancies are responsible.
In summary, the fully hydrogenated vacancy, ![]() , acts as a
single donor due to a partially filled singlet near the top of the
gap; thus
, acts as a
single donor due to a partially filled singlet near the top of the
gap; thus ![]() will compensate
will compensate ![]() in InP.
Removal of hydrogen atoms from the vacancy leads to increased acceptor
character as the triplet state starts to empty.
in InP.
Removal of hydrogen atoms from the vacancy leads to increased acceptor
character as the triplet state starts to empty.